There are several ways to adsorb tributyl phosphate on the mineral surface: electrostatic adsorption, chemical adsorption, surface precipitation, multi-layer adsorption and multi-layer accumulation. Since the amount of tributyl phosphate reaches a certain value, the free ions in the solution react with the Ca2+ and Sn4+ ions in the solution to cause hydrophobic adsorption. This hydrophobic adsorption is fundamentally chemical adsorption or surface deposition, and it is in all The interpretation of the mechanism of action of tributyl phosphate is dominant. However, Li Quan believes that in the presence of tributyl phosphate, fine cassiterite ore particles, especially those below –10 μm, will agglomerate. The interaction between the ore particles includes not only van der Waals and electrostatic interactions (DLVO interactions), but also other forces capable of agglomerating the particles. The addition of a flotation agent, particularly a collector or a hydrophobic agent, will produce a hydrophilic-hydrophobic interaction force in the slurry system that is one to two orders of magnitude greater than the electrostatic and van der Waals forces. It is this presence of force that leads to agglomeration between the ore particles and an increase in the sedimentation yield of the mineral. However, it does not mean that the greater the concentration of the collector or the hydrophobic agent, the stronger the hydrophobic agglomeration. When the amount of tributyl phosphate reaches a certain value, the mineral sedimentation yield decreases. On the one hand, this phenomenon should be attributed to the increase of the potential energy of electrostatic interaction between particles; on the other hand, the formation of precipitates occurs simultaneously on the surface of the mineral and in the solution, so that the residual precipitate in the solution increases, which is in the solution. The precipitate in the reverse adsorption on the mineral surface, the hydrophilic group extends to the solution, weakens the hydrophobicity of the mineral surface, enhances its hydrophilicity, and leads to a decrease in the mineral sedimentation yield. The addition of a certain amount of tributyl phosphate can enhance the agglomeration between fine cassiterite and, in combination with other cassiterite collectors, can improve the flotation recovery rate of fine cassiterite. 2 Mechanism of action of octyl hydroxamic acid on the surface of cassiterite Chemical analysis of the solution indicated that the pH range of the cassiterite recovery was octyl hydroxy citrate ion-molecular co-adsorption mode. The forces on the surface of the collector and cassiterite include chemical forces, electrostatic forces, and hydrogen bonding forces. The presence of octyl hydroxamic acid negatively shifts the zero point of the pure mineral of cassiterite and lowers the mineral potential. Infrared spectroscopy analysis shows that the interaction between cassiterite and octyl hydroxamic acid is mainly chemical adsorption, hydrogen bonding and electrostatic interaction. The reaction product can be expressed as the O, O-5 five-membered ring structure of Sn2+. When the concentration of octyl hydroxamic acid is more than 30 mg/L, it may form a multi-layer adsorption of the agent on the surface of the cassiterite. 3 Mechanism of action of benzoic acid and cassiterite surface When the pH of the flocsite is maintained at natural pH (ie, pH 6–7), the carbamazee is present in both aqueous form [HA] in aqueous solution, as well as [A–]hydroxamic acid anions. exist. And when the pH exceeds this range, its collection performance is greatly reduced; and at a low pH range, it is more severe than at a high pH. At pH 6.5, the localized ions of cassiterite are Sn(OH)3+ and Sn(OH)5−. May be present throughout the flotation process two different forms of action, on the one hand when cassiterite surface active metal cations Sn4 +, mainly by hydrolysis of the complex with tin hydroxyl hydroxylated SnO2 is formed by dehydration, Sn4 + It can form a chelate with [A–] hydrolyzed by benzoxamic acid to produce chemisorption; on the other hand, the non-polar group of [HA] molecule can be adsorbed on the surface of cassiterite by hydrogen bonding. For the entire recovery rate change, chemisorption should be the main role of the collector on the surface of the cassiterite. Infrared spectroscopy indicated that the N–H bond in the benzoic acid was destroyed during the adsorption process, and the adsorption was basically determined to be chemisorption. The benzyl hydroxamic acid molecule has two tautomers (benzoic acid and benzohydroxamic acid) in aqueous solution. When it is treated with benzyl hydroxamic acid, the molecule can be completely converted into a component. Exist, and vice versa. The newly formed substance does not exhibit an N–H bond after the action. In addition, the broad and strong absorption peak at 3444.0cm–1 in the infrared spectrum may be generated by water molecules, or it may be the absorption peak of O–H stretching vibration associated with hydrogen bonding, that is, the collector cannot be determined at present. Whether physical adsorption occurs on the mineral surface. 4 Mechanism of action of new collector SR and cassiterite surface Li Quan applied infrared spectroscopy and Zeta potential measurement to study the mechanism of the new collector SR and the surface of cassiterite. In the range of pH > 4.5, the surface of the cassiterite is negatively charged. After the addition of SR, the negative charge value increases, and the zeta potential changes greatly at weakly acidic and neutral pH, and the zeta potential changes at alkaline pH. The main form of SR adsorption on the surface of cassiterite is not electrical adsorption, but is characteristic adsorption, because the anionic collector can adsorb on the surface of the negatively charged cassiterite and increase its electronegativity. The infrared spectrum of SR and pure mineral SnO2 has obvious characteristic peaks. At 1560 cm-1, there is a C=O double bond absorption peak, and the main absorption peak positions correspond to SR tin salt. In addition, the characteristic peaks of the minerals have changed, indicating that there are SR tin salt products on the surface of the cassiterite. Infrared spectroscopy indicates that the agent is chemisorbed on the mineral surface. 5 Mechanism of flotation of fine-grained cassiterite by combined collector The optimum pH of each collector for the flotation of cassiterite is different. There is a positive synergistic effect between the ZF agent and the secondary collector (TBP), and the use of the secondary collector (TBP) can promote the increased hydrophobic capacity of the ZF agent-mineral system. That is, the ZF collector forms a chelate on the surface of the mineral, making the mineral surface hydrophobic, but due to the insufficient hydrophobic ability of the chelate, the addition of TBP produces a poorly soluble and hydrophobic multilayer on the surface of the formed chelate. The cover allows the mineral surface to have sufficient hydrophobic capacity to float. Styrene-phosphonic acid and benzyl phthalic acid can achieve effective recovery of cassiterite only in a strong acid medium. Calcite maintains good floatability in the flotation pH range, while quartz does not substantially float (or has a low recovery). The results of potentiodynamic test showed that the addition of the combined collector could negatively shift the surface potential of the cassiterite, and the relationship between the surface potential and the pH environment of the solution was significant, and the relationship with the amount of collector was not significant. Infrared spectroscopy results show that the intrinsic absorption peak of cassiterite is displaced, and a new characteristic peak is formed on the mineral surface. The combination of C=O and P=O in the combined collector and Sn forms a multi-chelate complex with the agent. A large number of non-polar hydrocarbon chain groups exist on the surface of the SnO2 after the action, and it is the hydrophobic action of these non-polar hydrocarbon chain groups that causes the cassiterite to float and be sorted. Dou Yongping studied the mechanism of flotation of fine cassiterite by combined collectors, and pointed out that the pH value of different collectors has different effects on the floatability of cassiterite. Among them, the combination of ZF chelating agent and TBP is used. The best effect of flotation is. The amount of collector has a great influence on the flotation effect of cassiterite, and the amount of collector increases, and the trapping effect produced by it is more prominent. In general, if a collector of ZF chelating agent combined with TBP is used, the amount of ZF chelating agent is 50 mg/L, the amount of TBP collector is 300 mg/L, and the pH value is controlled to about 7.77. The flotation recovery rate of fine cassiterite can reach 88.79%. Under Water Extrusion Pelletizer
LINA Extrusion Pelletizer
Most of the polymer must be kneaded and then granulated before it is made into a final product. Sorted by pelletizing ways, LINA rubber & plastic granulator can be divided into four categories: 1. Hot-cutting and air-cooling pelletizing method. 2. Under-water granulating method. 3. Water-strand extrusion method. 4. Water-ring granulation method. LINA granulator can meet the granulation needs of high-viscosity and low viscosity rubber and plastic materials, as well as some new materials and chemical additives.
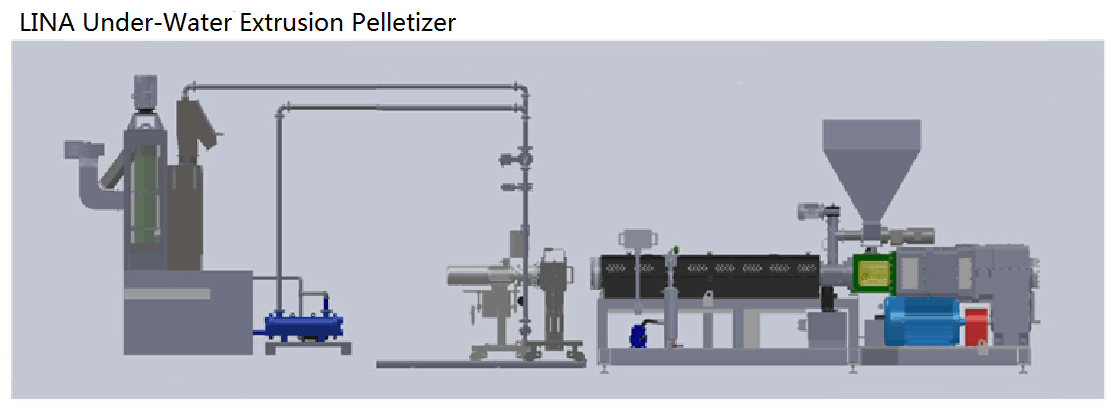
LINA Under-water Extrusion Granulator
LINA`s rubber and plastic under-water pelletizing machine is suitable for high-viscosity materials that may easily stick on the rotary cutter and are very sensitive to temperature, such as high viscosity copolymer elastomer (EVA, TPU, TPE, TPR), high viscosity hot melt adhesive and paraffin. This type of is actually similar to die surface hot cutting pelletizing method, the difference is, instead of cool air, there is a steady flow of water through the mold surface and the water directly contact with the die surface. The size of the pelletizing room is such that the blade is free to rotate over the die surface without limiting the water flow. The molten polymer is extruded from the die surface, and the pellets are brought out of the granulation chamber by means of the conditioned water to enter the centrifugal dryer.
Assembly Ling Diagram of LINA Under-Water Extrusion Granulator
Advantage of LINA Pelletizing Line
1. The unit has the whole unit process chain, sound and light failure alarm and fast lock fault point and other control functions. All parts that are in contact with the material are made of stainless steel.
2. High accuracy of temperature control system to ensure the temperature sensitivity of the material.
3. It adopts the drop type separating method after cutting process, to avoid Particle agglomeration and ensure particle cooling.
4. Feeding system of the pressing machine adopts heater and reversing device to solve problem of power outage, material crash and cleaning.
5. Especially, the barrel and die head adopts advanced foreign techniques to ensure and control the dangerous occurred when the pressure is uncontrollable. The honeycomb filter plate is easy to clean and quickly change the mesh and the die head t easy to leak.
6. The hoist adopts automatic return technology to ensure the working efficiency.
7. The technological combination of dual-pull forced feeder and single screw not only meet the high requirement of secondary continuous mixing, but also solve the problem of time and power consuming of traditional working process.
Specifications of LINA Extrusion Granulating Line
Name
Mode
Capacity of Kneader
Screw length to diameter ratio
Screw diameter
Dimension
LINA Extrusion Pelletizing Line
LN-10/70
10L
12:01
70mm
2200*1000*1150
LN-10/75
10L
12:01
75mm
2650*1100*1250
LN-35/100
35L
12:01
100mm
3800*1090*1690
LN-55/120
55L
12:01
120mm
3800*1090*1690
LN-75/135
75L
12:01
135mm
3850*2350*3280
LN-110/150
110L
12:01
150mm
5100*2000*1500
LN-110/165
110L
12:01
165mm
5100*2000*1500
Underwater Extrusion Pelletizer,Underwater Pelletizer,Pellet Machine,Plastic Pelletizing Machine LINA Machinery Industrial Co.,Ltd , https://www.linakneader.com
The principle of tin ore dressing agent and mineral surface
1 action tributyl phosphate flotation of cassiterite