INGENTAL is Pet Preforms for juice Bottle,38mm Neck Pet Preforms,26g Pet Preforms,Welcome Buy . Pet Preforms for juice Bottle,38mm Neck Pet Preforms,26g Pet Preforms Ningbo Ingenta Mold & Machinery Co.,Limited , https://www.china-ingenta.com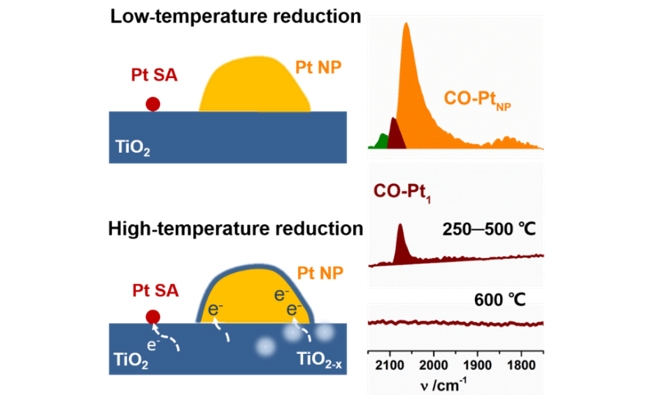
Strong metal-support interactions of single-atom catalysts discovered by Dalian Chemical
[ Instrument R&D of Instrumentation Network ] Recently, Qiao Botao, a researcher of the Catalysis and New Materials Laboratory of the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Zhang Tao, a member of the Chinese Academy of Sciences, have made new progress in the study of monatomic metal-carrier strong interactions. Strong metal-support interaction with the TiO2 support occurs, but the required temperature is higher than that of Pt NPs, and the reason why Pt single atoms lose their CO adsorption capacity is different from Pt NPs. It is not the physical coverage of the support, but the coordination saturation.
Atomic layer deposition (atomic layer deposition, ALD) is an advanced thin film deposition technology. Using the technical characteristics and advantages of ALD, a new type of high-efficiency nanocatalyst can be designed and synthesized, and the surface interface structure of the catalyst can be precisely adjusted. The research team led by Qin Yong, a researcher at the State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, used ALD technology to design and prepare a multi-domain Ni-based hydrogenation catalyst. Compared with the unconfined catalysts, the activity and stability of the multi-confined Ni-based catalysts for the hydrogenation of cinnamaldehyde and nitrobenzene have been significantly improved.
In 1978, Tauster et al. found that platinum group metals supported by reducible carriers would lose their ability to adsorb small molecules (CO, H2) after high-temperature reduction, and they were named SMSI effect. SMSI has a significant influence on the activity, selectivity and stability of the catalyst, which has caused widespread concern and in-depth research. In the previous research, Qiao Botao and Zhang Tao team and Wang Junhu team discovered various SMSI effects of gold and platinum group metal nanocatalysts. In the single-atom catalyst, the single atom acts as the active center, and it should be more affected by the interaction of the metal support than the supported nanocatalyst. However, whether the classic SMSI effect can occur on the single-atom catalyst is unknown.
The photochemical method is a method that uses light energy to activate a reactive substance to cause a chemical reaction. Referred to as the actinic method. After the photon is absorbed by the reactant, the electrons in the bond or non-bond orbit are excited to the anti-bond orbit, causing the bond to weaken or even break, which activates the reactant molecule. In order to increase the yield, the reactants must be continuously irradiated with light in a static system. Common devices include solution photochemical synthesis device and gas photochemical synthesis device. This method is applicable to the synthesis of certain metal carbonyls, metal organic compounds, boron compounds, etc.
When the photochemical reaction proceeds, the photon must have sufficient energy, and ultraviolet and visible light are commonly used as light sources. Second, the photons can be absorbed by the reactants. For example, the dissociation energy of fluorine is 153 kJ/mole, which corresponds to the energy of a photon with a wavelength of 780 nanometers. However, fluorine does not have an absorption band in the red band, but only has a strong The absorption band, therefore, when synthesizing xenon difluoride by the actinic method, it is not possible to irradiate with ultraviolet light or sunlight with red light. If the reactant itself does not have a corresponding absorption band, some substances can be added to achieve the goal by means of energy transfer. For example, mercury atoms can absorb photons with a wavelength of 253.7 nanometers, and can transfer energy through collisions between excited mercury atoms and reactant molecules to activate the reaction.
In this work, the research team prepared a Pt/TiO2 catalyst (where Pt monoatoms and NPs coexist) through improved photochemical methods. Under the same reduction conditions, NPs lose their CO adsorption capacity at 250 ℃, while monoatomic platinum species The CO adsorption capacity is lost only when the reduction temperature is 600 ℃. After oxidation treatment, the CO adsorption capacity can be restored. LEIS detection of Pt atoms on the surface before and after SMSI occurred. The results showed that after high temperature reduction, the surface Pt single atoms were neither embedded in the carrier nor encapsulated. Theoretical calculations indicate that the reason why Pt single atoms lose their CO adsorption capacity is coordination saturation (18 electron rule), not physical coverage. Based on this new discovery, using 3-nitrostyrene hydrogenation as a probe reaction, Pt NPs were selectively encapsulated by reduction treatment. The results proved that the single atom was the main active center in the reaction, and the nanoparticles contributed little.
This study found for the first time that the classical SMSI effect can occur in a single-atom catalyst system, and revealed that it is different from the nanoparticle SMSI. It not only helps to deepen the understanding and understanding of the SMSI effect, but also provides a new method for studying the catalytic active center and regulating catalytic performance. method.
Source: Encyclopedia, Dalian Institute of Chemical Physics