Positive Pressure Yibin Tianruida Auto Parts Co.,Ltd , https://www.trdautoparts.com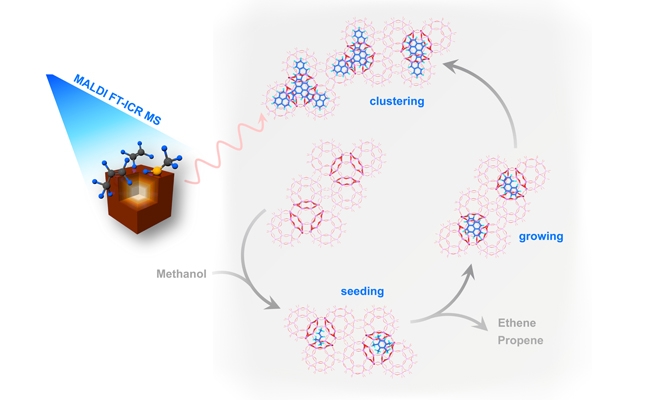
Molecular sieve catalyzed carbon deposition across cage growth mechanism discovered by Dalian Institute of Chemical Technology
[ Instrument Network Instrument R & D ] Recently, the team of Liu Zhongmin and Wei Yingxu of the Low Carbon Catalysis and Engineering Research Department of the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences made new progress in the deactivation of carbon deposits in the methanol-to-olefin reaction. Cage growth mechanism.
Molecular sieves are synthetic hydrated aluminosilicates (foam zeolites) or natural zeolites with the function of screening molecules. Its chemical formula is (M′2M) O · Al2O3 · xSiO2 · yH2O, and M ′ and M are monovalent and divalent cations such as K +, Na +, Ca2 +, Ba2 +, and the like. It has many pores with uniform pore size and neatly arranged pores in the structure. Molecular sieves with different pore sizes separate molecules of different sizes and shapes. According to different molecular ratios of SiO2 and Al2O3, molecular sieves with different pore sizes are obtained. Its models are: 3A (potassium A type), 4A (sodium A type), 5A (calcium A type), 10Z (calcium Z type), 13Z (sodium Z type), Y (sodium Y type), sodium mordenite type Wait. It has high adsorption capacity, strong selectivity and high temperature resistance. Widely used in organic chemical industry and petrochemical industry, it is also an excellent adsorbent for gas dehydration. Attention has also been paid to exhaust gas purification.
Industrial processes such as petrochemicals (catalytic cracking, isomerization, etc.) catalyzed by molecular sieves, and coal chemical industries (methanol to olefins, synthesis gas conversion, etc.) have important positions in the national economy. Due to their acid catalytic characteristics, the catalyst is lost due to carbon deposits. Living is common. The catalyst needs to be continuously burned to regenerate the charcoal to maintain its activity, so as to realize the long-term operation of the device. Although the problem of carbon deposition and deactivation of molecular sieve catalysts has been paid much attention, how to prevent the catalyst from carbon deposition is still a long-term scientific challenge. To solve this problem, a deep understanding of the mechanism of catalyst carbon deposition deactivation is required. At present, the literature's understanding of molecular sieve carbon is summarized as simple condensed-ring aromatic hydrocarbon species inside the molecular sieve or graphitized carbon deposits generated on the outer surface of the molecular sieve. It is difficult to correlate the exact carbon evolution path. It is of great practical significance to analyze the structure of carbon deposition species at the molecular level and track the evolution of carbon deposition species for carbon deposition deactivation and catalyst regeneration.
An olefin refers to a hydrocarbon containing a C = C bond (carbon-carbon double bond) (olefinic bond). They are unsaturated hydrocarbons and are divided into olefins and cyclic olefins. According to the number of double bonds, they are called monoolefins and diolefins. One of the double bonds is a π bond with higher energy, which is unstable and easily broken, so an addition reaction occurs. The single-chain olefin molecule has the general formula CnH2n, and C2-C4 is a gas at normal temperature. It is a non-polar molecule and is insoluble or slightly soluble in water. A double bond group is a functional group in an olefin molecule and has reactivity. It can undergo addition reactions such as hydrogenation, halogenation, hydration, halogenated hydrogenation, hypohalous acidification, sulfation, epoxidation, and polymerization. It can also oxidize double bonds. The cleavage of aldehydes, aldehydes, carboxylic acids, etc.
In this study, the research team combined the conversion of methanol to olefins, and used a combination of high-resolution mass spectrometry and isotope labeling technology, combined with theoretical calculations, to successfully analyze the previously unknown high-molecular complex carbon deposit molecular structure, and systematically studied the product. During the carbon growth process, the cross-linked growth process of the initial carbon deposition primitive species between the molecular sieve cages gives a relatively complete evolution path for the carbon deposition of the methanol-to-olefin reaction: SAPO-34 molecular sieve cages gradually expand and thicken the active hydrocarbon pool species. Cyclization generates 3 to 4 ring carbon deposit precursors, and these carbon deposit structural elements are then cross-linked across the cage through covalent bonds to form polynuclear, nanographene-like fused ring aromatic hydrocarbon species. These cross-cage cross-linked fused aromatic hydrocarbon species severely hinder the reaction mass transfer and cause catalyst deactivation. Subsequent expansion studies found that cross-cage carbon inactivation behavior is also common in other cage structure molecular sieve catalytic systems. The discovery of the deactivation mechanism of cross-cage carbon deposition will not only promote the in-depth study of the basic principles of methanol conversion reactions such as the methanol conversion network and the cage control principle, but also provide a theoretical reference for the design of efficient catalysts and the optimization of the char burning regeneration process for industrially deactivated catalysts .
Source: Dalian Institute of Chemical Physics, Encyclopedia