Edible gelatin is widely used as a thickening agent in the food processing industry. If it is frozen, food coloring, fudge, ice cream, cool, yoghurt, frozen food, etc., therefore, the safety of gelatin and people's health is extremely high. close relationship. Bleaching agents such as sulfites and their salts are often added during the production of gelatin. The most unwanted pollutant in this bleach is sulfur dioxide. At present, there are two methods for determining sulfur dioxide and sulfite in foods stipulated by the national standard law: hydrochloric acid. The national standard method of rose aniline method and distillation method specifically targeting sulfur dioxide in gelatin is a glass distillation method. This method requires a custom-made glass apparatus, and the distillation takes a long time. Root wood cannot adapt to the detection of batch samples in the inspection industry. It has also been reported in the literature that the use of direct distillation to determine the sulfur dioxide content of gelatin has eliminated the requirement for complete distillation apparatus in the national standard, but the distillation efficiency has not been significantly improved, and the direct heating method of the heating source temperature is not well controlled. polluted environment. In this paper, the Kjeldahl nitrogen analyzer is similar to the distillation method in that the Kjeldahl nitrogen analyzer is used for the determination of sulfur dioxide in gelatin without any configuration change of the instrument, and the distillation time is greatly shortened (<10 min). Good experimental results, method accuracy and accuracy meet the requirements. Dandong Nondestructive Testing Equipment Co.,Ltd manufactures Industrial X ray complete series products from beginning of 1988. built our own X Ray Tube production line for years, use our own tube on all X ray products. based on complete strict testing in our own lab before delivery to all customer, we get very good feedback and professional leading position. X Ray Tube X Ray Tube,X Ray Tube Parts,Tube X Ray,Modern X Ray Tube Dandong Nondestructive Testing Equipment Co., Ltd. , http://www.ddchinaxray.com
1 Experimental section 1.1 Principle The sample was acidified and well distilled in a closed vessel. Sulfur dioxide was distilled off. Diluent was absorbed by hydrogen peroxide solution and then titrated with sodium hydroxide standard titrant according to the consumed sodium hydroxide. The standard titrant calculates the sulfur dioxide content.
1.2 Reagents and materials Sulphur dioxide standard solution (purchased from National Standards Center), sulfuric acid (1+4), 30% sulfur dioxide, (1+9) sodium hydroxide titration solution (0.025 mol/L, calibration required), methyl The red-methylene blue stick is a small agent: 0.5 g of methyl red and 0.33 g of methylene blue are dissolved in 200 mL of ethanol. Article reagents are analytically pure except as noted.
1.3 Instruments and Equipment Semi-automatic Kjeldahl distillation unit KT260 distillation unit (Danish Flowserve), Kjeldahl distillation tube, 250 mL accept bottle, titration stand, basic burette.
1.4 Experimental Procedures and Results Calculation About 5.0 g of gelatin powder is placed in a 100 mL beaker, 50 mL of distilled water is added to make it completely swollen. Then it is transferred to the Kjeldahl nitrogen distillation tube and rinsed with 50 mL of distilled water for a small number of times. Beaker, lotion is also transferred to the distillation tube. Distillation s}:: Installed on Kjeldahl, rotate it to complete a seal. Place 20 mL of hydrogen peroxide absorbing solution (1+9) in the receiving flask and neutralize it to the end point (add 2 drops of methyl red-methylene blue stick to the hydrogen peroxide solution, the solution is light purple, The color was grass green when neutralized to the end with sodium hydroxide solution). The receiver tube was placed in a receiving flask and the well was submerged in hydrogen peroxide solution at its lower end. Press the acid pump, add 20 mL of sulfuric acid (1+4), then turn on the distillation switch and heat distillation. When receiving about 120 mL of the bottle, allow the receiving bamboo to leave the liquid surface, distill for another 1 min, close the distillation switch, rinse the outside of the lower end of the receiving tube with a small amount of distilled water, remove the receiving bottle, and add 1 drop of the fingering agent, using the hydroxide The sodium standard solution was titrated until the color was grass green, and it did not fade within 30 s.
Remember the amount of sodium hydroxide solution consumed. Use the same steps to make a blank experiment.
The formula for calculating the result is: X=c×(V-V0)×0.064×0.5×10/m
In the formula: X - the content of sulfur dioxide in the sample, mg/kg; c - the molar concentration of the standard sodium hydroxide titration solution, mol/L; V - the volume of the sample consumed sodium hydroxide solution, mL; V0 - the blank consumption of the hydroxide Volume of sodium solution, mL; millimolar value of 0.064-sulfur dioxide, mg/mol; m-mass of weighed sample, g.
2 Results and Discussion 2.1 Selection of Absorption Solution The researchers used acetic acid as the absorption solution, but acetic acid was a toxic substance. Scabblycin is a recognized environmental pollutant, and prolonged exposure to a large number of substances can easily cause mispoisoning. The article uses hydrogen peroxide solution as an absorbent according to the national standard method, which is environmentally friendly and does not produce toxic products. The effect of different hydrogen peroxide concentrations (1:19, 1:9, 3:17) on the results was compared and it was found that the hydrogen peroxide concentration was too low (1:19) to affect the absorption of sulfur dioxide, resulting in low results; Hydrogen peroxide (1:9) and high concentration of hydrogen peroxide (3:17) did not have any difference in HV. In this paper, hydrogen peroxide (1+9) was used as an absorbent.
2.2 Selection of sample weight The same gelatin was weighed in 3 g, 5 g, 8 g, and lOg for 6 determinations respectively. The statistical results were small. When the sample volume was small, the results showed a large error. When the sample volume was large, it tended to condense when swelled. And due to the limited capacity of the Kjeldahl distillation tube itself, too much swelling gel makes distillation difficult, resulting in no complete distillation. Therefore, it is better to take 59 for testing.
2.3 The choice of the amount of distillate is called the same gelatin 5.0 g determination, when the distillate is 120 mL, 150 mL when the distillation is stopped, after six repetitions of statistical determination, calculated by t test t = 1.04, less than t ( T0.05, 5) = 2.57, there is no difference in HV between the two, so the selected distillate was 120 mL.
2.4 The precision test of the method was determined by the wooden method using gelatin from Henan Jiaohe (No. 1) and gelatin from Hangzhou, Zhejiang (No. 2). Each sample was measured six times. The results are shown in Table 1. 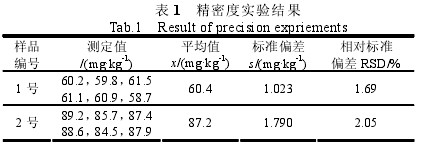
Table 1 Accuracy Test Results 2.5 Accuracy of the method Experiments were performed by adding 10 parts of a 5.0-gram gelatin sample for recovery. Five of these were added with 50 ug of sulfur dioxide standard and the other five were added with 100 ug of sulfur dioxide standard. The determination of sulfur dioxide content, measurement results and recovery experiments are shown in Table 2. 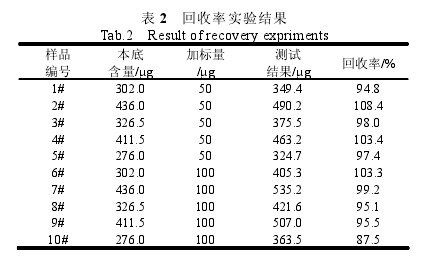
Table 2 Test results of recovery 2.6 Comparison experiments Take 5 different samples, respectively, with a glass distillation instrument and Kjeldahl determination of sulfur dioxide content was repeated six times, the results are shown in Table 3. After t-test calculation, t=1.75, t(0.05,5)=2.57, t3 Conclusion The semi-automatic Kjeldahl distillation unit has achieved good results for the determination of sulphur dioxide in gelatin. The instrument can be modified without any modification. It is used directly for testing (3~5 cycles of top heat after start-up). It takes 5~7 minutes for each sample to be analyzed, which greatly shortens the distillation time and improves the detection efficiency. This method has the advantages of easy operation, high precision, high accuracy, and no significant difference with a glass distillation instrument, which expands the application range of Kjeldahl nitrogen analyzer and can save the testing cost for enterprises and institutions. 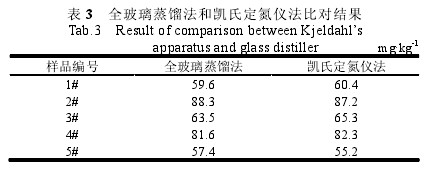
Table 3 Comparison of glass distillation and Kjeldahl method